- Key growth drivers continue to show positive results. Ilumetri® performed strongly despite a decrease in new patient initiations during the COVID-19 pandemic, while Skilarence® results matched expectations, with flat sales excluding compounding impact in The Netherlands. Seysara® TRx decline is stabilizing following the impact of COVID-19, which disproportionally hit the acne market in the US, and new prescriptions began to recover in June.
- Innovative pipeline progress with significant mid-term value still to be unlocked. Tirbanibulin (actinic keratosis) launch in the EU and US is expected in early 2021. FDA approval of inclusion of wording in the Seysara® Microbiology labelling, an important achievement for the relaunch. Lebrikizumab (atopic dermatitis) expected launch in 2023 remains on track, visibility continues to increase compared to competitors.
- In June, Almirall was selected by the IBEX Technical Advisory Committee (CAT) to join the IBEX 35, the benchmark Stock Market index of the Bolsa de Madrid, Spain's principal stock exchange.
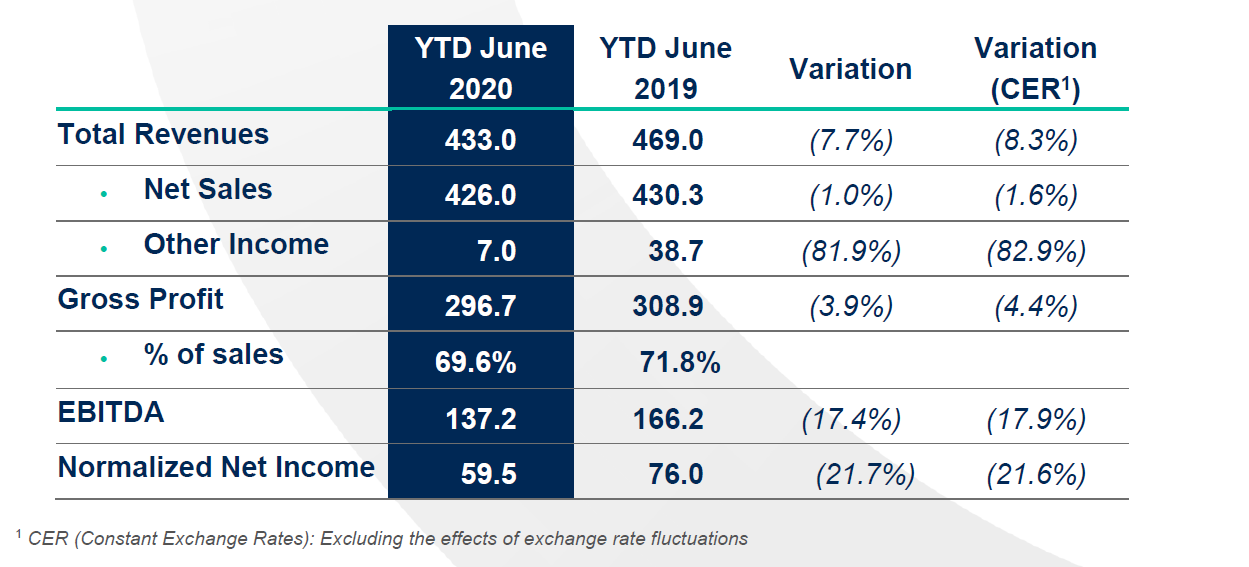
- Q2 Business performance impacted in H1 by COVID-19 and genericization of Aczone®: Net Sales €426.0 MM -1% (+6% ex-Aczone®), Total Revenues: €433.0 MM, -8% and EBITDA reached €137.0 M, -17%. FY 2020 EBITDA Guidance revised to €230 - €250 MM with lower net sales expectations to reflect updated impact of COVID-19.
Sustained performance of key growth drivers and excellent progress on pipeline in H1 despite negative impact of COVID-19
27 July 2020Financial highlights (€ million)
The global COVID-19 pandemic continues to evolve, forcing Almirall to face an unprecedented and challenging scenario, which also varies across regions, increasing its complexity. The company has managed to respond to this situation in a manner that has limited its impact on operating results, maintaining stability in an otherwise unstable period. Since the beginning of the crisis, we have focused our efforts on offering a strong response to the needs of the sector and I am particularly proud of our employees, who have maintained the production of medicines despite the pandemic to fulfil our commitment to our patients.
As part of the biopharma industry, we have offered our help to overcome the crisis and protect public health, working alongside health professionals, whom we sincerely thank for their commitment to curb the virus and protect us all.
The dermatology business in the US and EU has been profoundly affected by the COVID-19 pandemic. The reduction in the number of patient visits due to lockdown and the change in prescription habits have had a negative impact on our results for the first half of the year. This fact, together with Aczone® facing generic competition, has caused a decrease in our Net Sales. We have adapted our commercial models in response to this new reality, allowing HCPs to keep in contact with our teams through virtual means.
Despite these challenges, our key growth drivers have continued to make progress. With Ilumetri®, we have seen positive sales momentum, despite a decrease in new patient initiations due to COVID-19. Our pipeline remains strong, following the execution of exciting deals, such as those announced in January with Bioniz and 23andMe, and the expected global launch of Tirbanibulin in early 2021, as well as the initiation of Phase III trials for Seysara in China later this year. These assets, together with Lebrikizumab, have the potential to deliver an exciting new era of growth for Almirall. Supported by this positive long-term outlook, we continue to focus on developing a portfolio of differentiated products, building the value of our pipeline, and prioritizing strategic opportunities to address patients’ unmet needs.
Finally, I am delighted that Almirall recently joined the IBEX 35, which offers greater visibility to the company and important growth prospects. This significant milestone shows the interest of the financial markets in granting the health sector the place it deserves, which will be crucial in the aftermath of this crisis.
Peter Guenter, CEO.
Almirall, S.A. (ALM) the global biopharmaceutical company based in Barcelona, has announced its HY 2020 financial results.
Summary results
- Net Sales and Total Revenues performance impacted in Q2 by COVID-19 and generic competition to Aczone in the US, at €426 MM and €433 MM, reflecting declines of 1.0% and 7.7% respectively.
- Gross Margin stood at 69.6%, a decrease of 220 bps compared to previous year, as expected, related to the genericization of Aczone®.
- Research & Development costs were €40.8 MM in HY, or 9.6 % of Net Sales.
- Sales, General & Administrative at €186.8 MM, a decline of 7.6% vs 2019, with a lower spending than forecasted on sales & marketing due to the impact of COVID-19 on commercial operations.
- EBITDA of €137.2 MM decreased by -17.4% (vs. 2019), adversely impacted by Aczone®
- genericization and COVID-19.
- Normalized Net Income of €59.5 MM reflected a decrease of 21.7% in comparison to HY 2019.
- Cash Flow from Operating Activities reached €68.1 MM.
- Shareholders Equity represented 45.5% of Total Assets.
Dermatology was the area most impacted by COVID-19
The pandemic has profoundly impacted the medical dermatology area. During the coronavirus lockdown, patients’ consultations and prescription patterns were significantly altered. Doctors and hospitals have experienced a significant reduction in the number of visits, leading to meaningful decreases in new patients treated with biologicals and other non-life-threatening treatments.
This unique situation has forced dermatologists to adapt their behaviours: In the US, a change in OAB branded prescribing patterns by dermatologists to new patients in favour of generic doxycycline has been observed. In addition, a number of EU dermatologists switched existing psoriasis patients away from their treatments due to safety concerns, management of potential side effects, and the need to administer or pick up medication in hospitals.
A progressive return to normal of healthcare systems is expected as soon as countries begin emerging from their lockdowns. Patient visits to doctors and hospitals are starting to normalize, and some European countries are showing the first signs of recovery. Face-to-face interactions have started to increase.
Almirall has adapted its business in response to the pandemic, adapting its commercial activities and implementing new ways of working. A contingency plan was put in place immediately to minimize the risk associated with the health and well-being of all employees. The company has also reaffirmed its commitment to patients by maintaining operations of its factories, which have been running at full capacity throughout the entire crisis, to ensure the supply of medicines to each patient who needed to be treated.
Compelling assets to lead medical dermatology in the future
Although the business performance was impacted in Q2 due to COVID-19 and the genericisation of Aczone®, Almirall has a robust, strategic dermatology portfolio. The company remains well positioned through a solid balance sheet and is focused on additional external opportunities that could boost future growth.
Almirall's portfolio of growth drivers continues to underpin its performance. In particular, Ilumetri®, an anti-IL- 23 high-affinity humanized monoclonal antibody indicated for the treatment of adult patients with moderate to severe plaque psoriasis, continues to show good momentum. The anti-IL-23 class increased its market share, capturing 34% of the German market in May, and also overtakes IL-17's in new patient share for the first time, becoming the leading class within biologics for moderate to severe psoriasis.
Ilumetri® delivered a robust H1 performance in Europe, with sales more than doubling to €18 MM. In Germany, the product registered the highest monthly unit volume in June since launch. Meanwhile, performance of Ilumetri® in Switzerland and Austria has recovered to pre-COVID levels, while the UK and Spain show a recent positive trend similar to the beginning of this year, despite having been heavily impacted by COVID-related measures.
Almirall recently obtained the price and reimbursement of Ilumetri® in France, a significant step that will mark the introduction of its medical dermatology portfolio in that country, the second largest psoriasis market in Europe. Launch is expected in September. Ilumetri® reimbursement across the EU has made good progress, and the company is working on further launches.
Skilarence®, an oral-systemic formulation for the treatment of patients with moderate to severe chronic plaque psoriasis showed, as anticipated, decreased revenues due to COVID-19 because of blood monitoring requirements and a lack of new patient initiations. Net sales remained flat year-on-year, excluding compounding impact in The Netherlands. A more gradual increase in sales is expected, moving forward as Dimethyl Fumarate-naïve countries require more time and education to achieve market penetration.
Seysara®, an innovative oral antibiotic derived from tetracycline and specifically designed to treat acne in patients over the age of 9 years, stabilized prescription volume. COVID-19 has severely impacted the US acne market, so that H1 Net Sales of Seysara® decreased 19% to €7 MM vs. 2019. In June, new brand prescriptions started to increase, which supports the company’s expectations of regaining market share once the COVID-19 crisis starts to normalize.
In June, the FDA approved an updated label for Seysara® which indicates that the primary bacterium associated with acne (P acnes) has shown limited potential for developing resistance to sarecycline (the active ingredient of Seysara®). This decision represents an important achievement for the relaunch of this product.
In addition, plans to submit Seysara® to the Chinese NMPA by 2023 are on track. It is estimated that there will be 13 million potential patients treated for moderate to severe acne among China’s urban population by 2028, creating a significant opportunity to launch an innovative product whose clinical development is mostly de-risked. Seysara® is Almirall’s first dermatological product to enter the Chinese market, a key milestone to begin building the company’s strategic dermatology portfolio in China. To demonstrate the drug’s efficacy and safety in China, Almirall will initiate a Phase III trial later this year.
R&D advances and reinforcing the pipeline with the latest agreements
Almirall’s more than 50 years of R&D experience translates into the number of solutions it has launched to the market in several therapeutic areas over the years. The company continues to work on expanding its R&D pipeline to make a real difference for patients and healthcare professionals. Its pipeline is progressing and has significant mid-term value potential.
In 2019, Almirall exercised its option with Dermira (which was subsequently acquired by Eli Lilly) to licence the rights to Lebrikizumab in in Europe for atopic dermatitis. Lebrikizumab is an anti-IL-13 monoclonal antibody that is currently in Phase III clinical development to assess its safety and efficacy as monotherapy in patients from 12 years of age with moderate to severe atopic dermatitis. Almirall believes Lebrikizumab has best-in-class potential and offers an opportunity to improve efficiency, tolerability, and convenience for patients. Almirall expects potential peak sales for Lebrikizumab in Europe of around €450 MM.
Regulatory filings for Tirbanibulin (actinic keratosis) have been completed and the product is under registration in the EU and the US, with launch estimated for the beginning of 2021. Tirbanibulin is a topical drug for the treatment of actinic keratosis in adult patients developed by Athenex and licensed by Almirall. The FDA and EMA filing are based on the analysis of two-Phase III studies (KX01-AK-003 and KX01-AK-004) that evaluated the efficacy and safety of Tirbanibulin ointment 1% in adults with actinic keratosis on the face or scalp. Tirbanibulin met the primary endpoint of complete clearance of actinic keratosis lesions on day 57 within the face or scalp treatment areas, with each study achieving statistical significance (p<0.0001) on this endpoint.
Almirall also signed several agreements to reinforce its pipeline in the first half of 2020, including deals with 23andMe and WuXi Biologics, and an option agreement with Bioniz Therapeutics Inc.
The strategic deal with Bioniz Therapeutics Inc. allows Almirall to augment its R&D pipeline with new treatment modalities to address highly underserved diseases within oncodermatology and immunodermatology, such as cutaneous T-cell lymphoma (CTCL), a rare type of non-Hodgkin’s lymphoma that affects the skin.
A recent interim analysis of Bioniz's Phase II clinical trial in CTCL shows highly encouraging efficacy and safety data. About 80% of subjects showed improvement in tumour burden as assessed by the modified severity weighted assessment tool (mSWAT) score in the absence of any concomitant therapy, of which approximately half achieved a 50% reduction or more in mSWAT score (defined as "partial response"). Almirall expects to make a decision on option exercise in Q4 2020. If it chooses to exercise the option, Almirall plans to start a Phase III trial in CTCL in 2021, with the aim of securing launch as early as 2023.
Guidance for 2020
Net Sales: low to mid-single-digit decline.
EBITDA: between €230 - 250 MM.
Investor Calendar 2020
- 9M 2020: November 9th
About Almirall
Almirall is a global biopharmaceutical company focused on skin health. We collaborate with scientists and healthcare professionals to address patient’s needs through science to improve their lives. Our Noble Purpose is at the core of our work: “Transform the patients' world by helping them realize their hopes and dreams for a healthy life”. We invest in differentiated and ground-breaking medical dermatology products to bring our innovative solutions to patients in need.
The company, founded in 1943 and headquartered in Barcelona, is publically traded on the Spanish Stock Exchange and is a member of the IBEX35 (ticker: ALM). Throughout its 77-year history, Almirall has retained a strong focus on the needs of patients. Currently, Almirall has a direct presence in 21 countries and strategic agreements in over 70, through 13 subsidiaries, with about 1,800 employees. Total revenues in 2019 were 908.4 million euros.
For more information, please visit almirall.com


