Innovation
Milestones in 2024
Go back to Growing the business & Innovating
Advancing our medical
dermatology pipeline
Roll out of Ebglyss® (lebrikizumab) across Europe
We successfully rolled out Ebglyss® (lebrikizumab) across Europe, making it available in 10 markets. Lebrikizumab achieved 34% growth in Q4 compared to Q3, driven by significantly increased sales, mainly in Germany, the largest dermatology market in Europe. Additionally, our collaborator, Eli Lilly, received FDA approval for lebrikizumab on 13 September, leading to a subsequent launch in the US market, while Swissmedic approval for lebrikizumab was granted on 30 August.
Ilumetri® (tildrakizumab) 2ml PFP EMA dossier’s submission and POSITIVE study
The Ilumetri® (tildrakizumab) 2ml PFP EMA dossier was submitted by the end of January and received positive feedback on 16 May 2024. Additionally, POSITIVE, a real-world evidence study, reinforced the impact of psoriasis on patients’ well-being showing that its burden is comparable to that of other severe diseases such as breast cancer. Results from this study showed that tildrakizumab can significantly improve patients’ quality of life, reaching levels comparable to the average population.
FDA approval of Klisyri® (tirbanibulin) for expanded area
Klisyri® (tirbanibulin) received FDA approval on 7 June 2024, for the treatment of actinic keratosis on an expanded area of the face or scalp (up to 100 cm²), providing visible progress in our quest to deliver impactful treatments for patients. The launch for this expanded indication in the United States in August gave patients and the medical community swift access this new treatment option.
To expand the label and include large field application also in Europe, a current clinical phase III study is aimed at enabling approval for an anticipated launch in 2026.
Regulatory success of Jublia® (efinaconazole)
We achieved Jublia® (efinaconazole) regulatory approval with the end of the decentralised regulatory procedure (letter End of Procedure (EoP) confirmation) from BfArM on 2 October, paving the way for upcoming launches across Europe. Based on this, Italy granted national authorization in December 2024.
Progress in the development of Interleukin-1 Receptor Accessory Protein
We completed the single and multiple ascending doses in healthy volunteers of our high-affinity monoclonal antibody targeting IL-1RAP (Interleukin-1 Receptor Accessory Protein) for the treatment of autoimmune dermatological diseases. This first-in-class, fully human monoclonal antibody has the potential to address unmet needs in several autoimmune dermatology indications.
Advancements in IL-2muFc Fusion Protein
A Phase I Multiple Ascending Dose (MAD) study for IL-2muFc Fusion Protein was initiated on 24 June. This molecule is designed to activate regulatory T-cells and has the potential to rebalance the immune system in several autoimmune diseases. This aligns with our commitment to innovation and developing ground-breaking solutions for patients with important and currently underserved dermatological conditions.
Fostering Innovation
& Strategic Partnerships
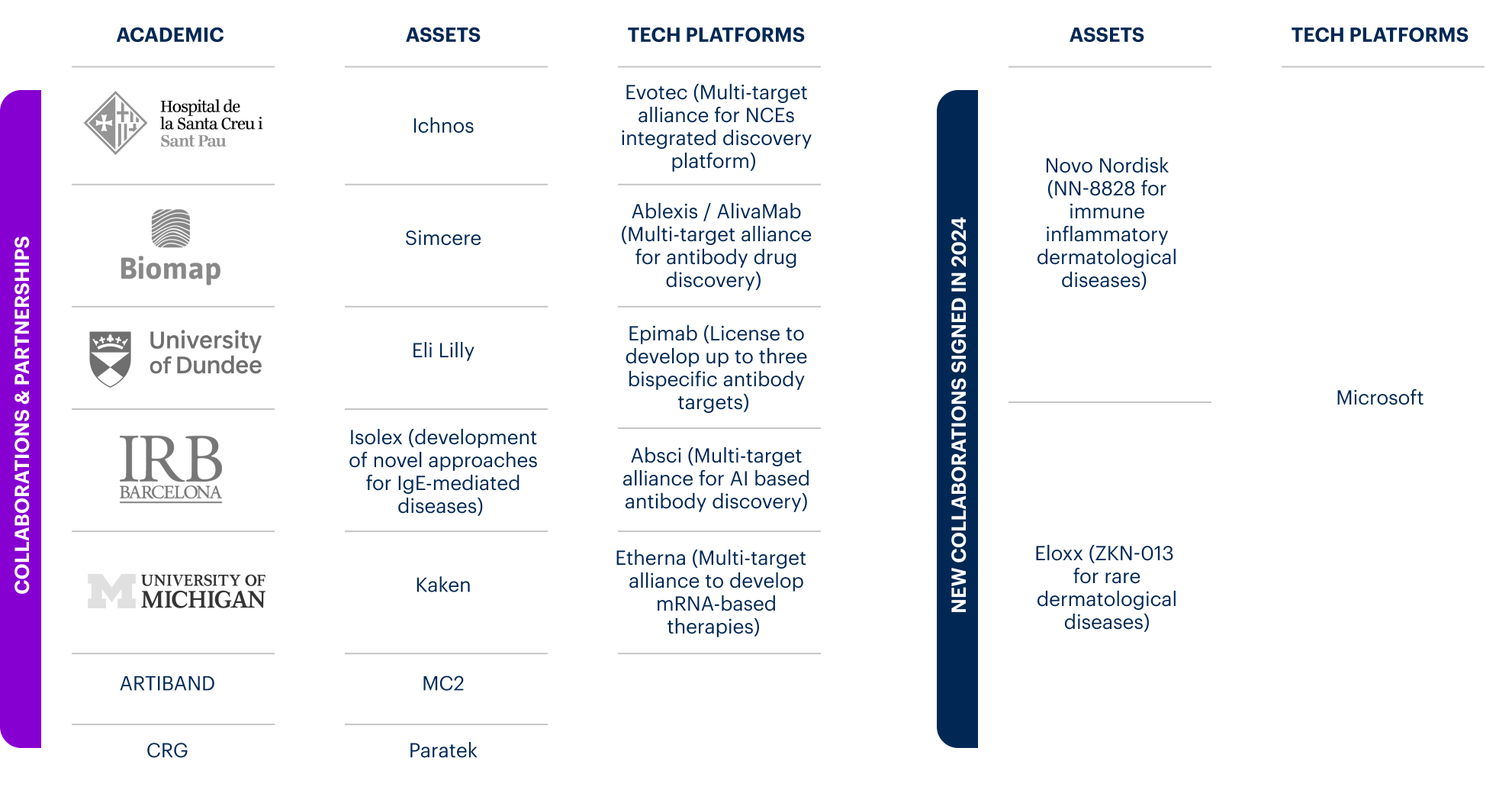
As part of our R&D expansion strategy, we entered several licence agreements in 2024 to strengthen our progress in dermatology treatments. This includes an anti-IL-21 monoclonal antibody (ALM401) which we entered an agreement for with Novo Nordisk on 16 February 2024, and an exclusive partnership with Eloxx Pharmaceuticals on 13 March to license ZKN-013, a novel treatment for rare dermatological diseases.

On 31 January 2024, we announced a novel partnership with Microsoft aimed at applying AI and advanced analytics technology to identify innovative opportunities within our pipeline portfolio.

On 8 February, we announced to join forces with the Centre for Genomic Regulation (CRG) to identify biomarkers to build new disease models for atopic dermatitis which will lead to the development of new treatment options.

The anti-IL-21 monoclonal antibody blocks the IL-21 cytokine, involved both in B- and T-cell biology and likely to be involved in several immune-mediated skin diseases.

ZKN-013 is an oral readthrough inducer designed to overcome nonsense mutations that cause a premature stop codon. ZKN-013 has promising potential in several rare indications. The phase I clinical study with ZKN-013 in healthy volunteers is currently ongoing.
Laying the foundation for future innovation in life sciences and medical dermatology
Almirall’s Innovation Hub
THE HIVE
Commemorating our 80th anniversary in 2024, we launched Almirall’s Innovation Hub The Hive (through an anniversary event at our Barcelona R&D facilities on 4 December in the presence of several institutional leaders and representatives of local, regional and national authorities). The Hive is a novel innovation hub held that is bringing together leading experts from companies such as ZeClinics, Centrient Pharmaceuticals, ADmit Therapeutics, and Microomics. The aim of this novel collaboration approach is to accelerate ground-breaking discoveries in life science and dermatology. Importantly, The Hive opens opportunities for further collaborations in the future.
OUR FOCUS ON INNOVATION
Continuously investing in advancing cutting-edge science and innovation throughout the years, we remain committed to our advancements in science and progressing our medical dermatology pipeline in the future.
In 2024, our progress was mostly driven by our sustained investment in R&D - at 12.6% of net sales. Our medical dermatology portfolio currently encompasses more than 50 products in different modalities, with a strong position in the area of immune-mediated inflammatory diseases. Our long-term approach to sustained investment in R&D, novel technologies, AI-based medicine discovery and product development, has resulted in a pipeline that is well positioned to provide sustained growth.
Through internal research and development, external partnerships and strategic in-licensing capabilities, we continue to expand our opportunities in R&D, and have become a scientific leader in medical dermatology. Our teams, located in our Barcelona innovation hub and closely connected to leading experts around the world, continue to advance in skin science and knowledge, leveraging high-impact treatments for patients worldwide.
We remain focused on pushing the boundaries of science and dermatology through innovation and research, improving patient outcomes with a patient-centric approach to product development.
PIPELINE
At Almirall, we believe in building collaborations to advance science and create a strong pipeline across the clinical and preclinical stages in key areas of medical dermatology. In 2024 we focused on collaborations and licensing agreements with partners that offer novel platforms and technologies that enable us to further develop innovative solutions for patients. Throughout the year, we conducted several Phase I studies in parallel with progressing our late-stage pipeline. Thanks to these efforts and our collaborations, we have access to latest available technologies, including small molecules, biologics and other modalities such as mRNA/LNP – accelerating the discovery and development of new therapies that address unmet needs in medical dermatology.
4 programs in Phase I with 4 PoC studies planned to start in the next 15
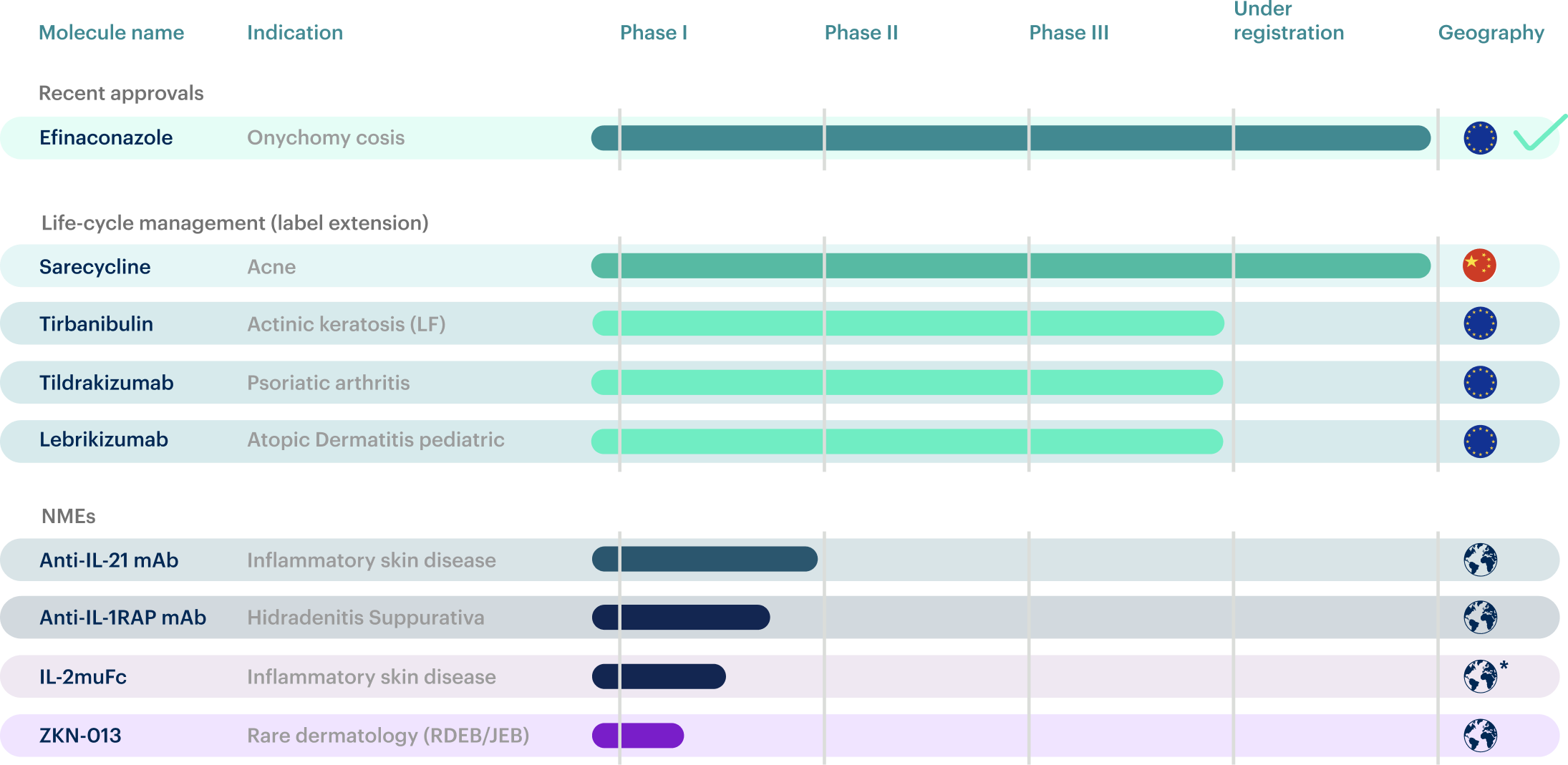
*Worldwide ex-Greater China
RDE B / JEB – Recessive Dystrophio Epidermolysis Bullosa / Junctional Epidermolysis Bullo sa
Collaborations & partnerships
At Almirall, we are committed to improving the quality of life of people with skin diseases by advancing science in medical dermatology, and by creating meaningful collaborations that lead to disruptive innovation.
Our patient-centric approach and dedication to cooperation have led to initiatives such as ‘Red Thread Connections’ which is aims at driving patient understanding, and supporting meaningful patient-dermatologist dialogues, and interactions with leading experts and institutions. These initiatives have delivered a range of impactful outcomes, including a patient-focused campaign for the treatment of AK. Studies like PROSES and PROAK open up new opportunities to build a better understanding of patients and treatments which we have presented at a range of scientific and medical conferences. By continuously reflecting on our efforts and creating new resources, we develop innovative treatments to enhance patients’ lives. Working alongside leading experts and dermatologists is a key part of our commitment at Almirall to shape the future of medical dermatology and patient outcomes.